Illustrative example - CRASH trial
The study computer will then randomly assign a treatment pack number that will identify one of the CRASH treatment packs stored in the emergency department.
CRASH treatment pack contains:
-
11 x 2g vials of methylprednisolone (MP) or placebo
-
1 x 20mL sterile water for injection (for use with the loading dose)
-
1 x 100mL bag of 0.9% NaCl (for use with the loading dose)
-
CRASH stickers (for attaching to infusion bags and patient notes)
-
Patient information leaflet and Early Outcome Forms
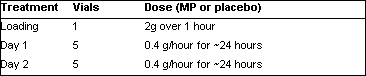
Loading
2g MP (or matching placebo) over 1 hour in 100mL infusion:
-
Add 20mL water for injection to one 2g vial and mix well
-
Add contents of vial to the 100mL bag of 0.9% NaCl provided
-
Infuse over one hour
Daily Maintenance
0.4g/hour for about 24 hours in 20mL/hour infusion (MP or matching placebo):
-
Remove 100mL from a 500mL bag of 0.9% NaCl (to make room for the steroid)
-
Add 20mL water for injection to each of five 2g vials and mix well
-
Add all five (total 100mL) to the 500mL bag of 0.9% NaCl
-
Infuse at 20mL/hour for about 24 hours
-
Repeat for maintenance day 2
N.B. As children under 16 are excluded, a simple fixed-dose treatment
can be used. The dosing regimen is that used in the NASCIS-2 and
NASCIS-3 trials of MP in acute spinal cord injury. (CRASH Trial
- go to protocol)
 Specifying
interventions in a clinical
trial
Specifying
interventions in a clinical
trial